
Manuscript Formatting and Submission Requirements
HSR Author Instructions
- Aims and Scope
- Manuscript Formatting and Submission Requirements >>
- Editorial Policies and Ethical Considerations
- Article Preparation Support
- Assistance Upon Rejection
- After Acceptance
- Production, Proofs, and Posting
- Post Publication
- Editorial Office Contact Details
-
Once your materials have been prepared according to these Author Instructions (details below), submit them online at ScholarOne Manuscripts. Details on how to use ScholarOne are here. For help with submission, please contact hsr@aha.org. HSR does not charge submission fees. For initial submission and revisions, you may include text, tables, and figures in a single file but on final acceptance you might be asked to upload some items such as figures in separate files.
By submitting a manuscript to HSR or reviewing for us, your name, email address, affiliation, and other contact details that the journal might require will be used for the regular operations of the publication. Please review Wiley’s Data Protection Policy to learn more.
If you have a manuscript under consideration, wait for a decision on that manuscript before sending a second manuscript using similar data and methods (except by special arrangement).
HSR recommends that all authors use ORCID. Corresponding authors must have an ORCID profile and enter their number on submission.
-
HSR publishes the following types of papers in its regular issues.
- Research Articles focus on important questions involving health and health services. These papers may apply quantitative, qualitative, or mixed methods.
- Research Briefs make incremental contributions to the literature such as validations or extensions of previous work, single-setting studies (with an argument for the generalizability of findings to other sites), or descriptive studies based on well-known databases. A research brief is also appropriate for articles in which the essential message can be more succinct than a full-length Research Article.
- Methods Articles and Methods Briefs use the same format as Research Articles and Briefs but focus on the development of new methods and tools, the application of current methods in novel ways, and examination of the pros and cons of using different methods and tools. Also appropriate are studies on new datasets and examinations of their value or limitations for health services research. Methods articles may also introduce methodological approaches understood by one discipline to readers in other disciplines.
- Commentaries Commentaries are by invitation only because of space limitations. Commentaries are evidence-based papers of 1250-2500 words, at the editors' discretion. They either highlight key findings in an accompanying research article, helping readers better understand the strengths and limitations of the subject paper and placing it in a broader policy context, or are standalone papers that promote a particular perspective on a health services research or policy topic. Commentaries should be organized into thematic sections with subheadings. A figure or table may be included with editorial approval.
Though commentaries must be invited, authors are encouraged to submit a proposal for a standalone commentary for editors to consider for invitation. Send a summary of 300 words or fewer on the issue you wish to address to hsr@aha.org. Include the 2 or 3 main points you will make and a brief outline of the supporting evidence (references do not count toward the word limit). Provide all author names, titles, and affiliations. Proposals will be evaluated based on the topic's suitability for HSR, salience or urgency, novelty, and evidence-based nature of the perspective. Invited proposals will undergo review with no guarantee of acceptance.
This example may serve as a stylistic model. HSR has limited space for standalone commentaries (about one per issue), so many worthy commentaries may not be invited for this reason. Editors will notify authors by email with a decision about the proposed commentary
-
The table delineates some of the key requirements for the article types in Section 2.2. Details are in Section 2.4.
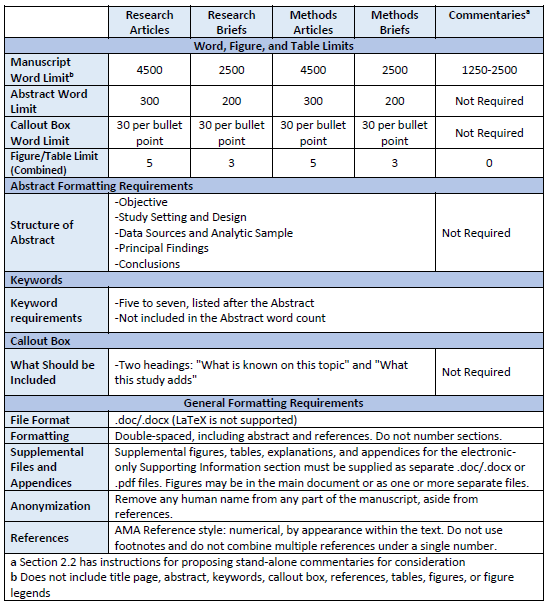
-
The subsections below provide detailed instructions on manuscript preparation. Please also refer to the Table in Section 2.3.
2.4.1 Cover letter
Please provide the following in a cover letter.
- Rationale for publishing the paper in HSR
- Statement about public disclosure of the manuscript’s contents, including previous or expected presentations or publications (peer-reviewed or otherwise) of the results. Though in general, HSR publishes original articles, we will consider work that has been disseminated previously through preprints, posted dissertations, or conference abstracts. (See Wiley preprint policy and Section 6.2). However, these must be disclosed in the cover letter at the time of submission, with a link if available. Please state if the submitted manuscript differs from the preprint or posted dissertation and if so, briefly describe how
- Rationale for using data that are all >5 years old, if applicable
- Description of any potential conflict of interest or presubmission approval requirement from funders or organizations providing in-kind support such as data access (Section 2.6)
2.4.2 Main manuscript files
As papers undergo double-anonymized peer review, the main manuscript text file should not include any information that might identify the authors or anyone else providing assistance (e.g., collegial comments on prior versions), including in the file name.
The main manuscript file for Research and Methods Articles and Briefs should have the elements below, (each of which is described further in subsequent subsections), presented in order.
- Title page with acknowledgments
- Structured abstract and keywords
- Callout box: What is known on this topic, What this study adds
- Main text
- References
- Tables and figures (each with title and footnotes for tables and legends for figures)
For review, we prefer that all these elements are in a single file. Please double-space all text and number all pages.
Commentaries require only the title page, commentary text, and references.
2.4.2.1 Title page with acknowledgements.The title page for the anonymized version of the uploaded manuscript should contain the following.
- A brief, informative title containing the major keywords. The HSR editorial team may edit titles for length and style.
- The title should not contain abbreviations
- With the exception of articles about methods, titles should not include information on methodology of the study
- Acknowledgments
- List all funding sources, including for the investigators' time and material support such as nonpublic datasets, or state "No funding to report." Authors are responsible for the accuracy of their funder designation. If in doubt, please check the Open Funder Registry for the correct nomenclature.
- To protect anonymity, for the version submitted for review, do not include individual names (e.g., people who provided feedback on drafts) or grant numbers.
- On acceptance, authors will update the Acknowledgments, naming individuals who contributed but do not meet the criteria for authorship, with their permission, and listing potential conflicts of interest or adding a statement that no authors have conflicts of interest.
- An accurate word count of the manuscript (not including abstract, keywords, callout box, references, tables and figure legends)
Important: In the "unblinded manuscript" slot, please upload a separate full title page with the information above as well as the following:
- Full names of the authors
- Authors’ institutional affiliations where the work was conducted with a footnote with a present address if different from where the work was conducted
2.4.2.2 Structured abstract and keywords. Manuscripts without appropriate abstracts will not be accepted (Commentaries excluded). Abstracts should not have citations. The following headings and information should be in the abstract (note the new abstract structure as of April 2024).
- Objective. Provide a single partial sentence, beginning with the word ‘‘To,’’ indicating the principal reason for conducting the study. For example: ‘‘To test’’ a specific hypothesis or theory.
- Study Setting and Design. Describe succinctly, in complete sentences, the study setting (e.g., type of health care systems, regions of focus, time span) and the general structure of the study methodology (e.g., descriptive, experimental, quasi-experimental, observational, qualitative). Describe interventions or exposures, participant recruitment or randomization, sample characteristics, diagnostic or therapeutic procedures. Identify the key outcome variables and other measures in the analysis.
- Data Sources and Analytic Sample. In complete sentences, indicate whether primary data were collected or secondary data were analyzed. Summarize key procedures used in assembling and analyzing the data, including eligibility and exclusion criteria, as appropriate.
- Principal Findings. Using complete sentences, focus on the most important observations from the data pertinent to the study. When possible, present numerical results (absolute numbers when available and rates only if not available) with appropriate indicators of uncertainty, such as confidence intervals. Do not report the results of statistical hypothesis testing alone, such as P values, which fail to convey important quantitative information. Reporting of odds ratios is discouraged (marginal effects preferred) except in case-control studies (see Norton and Dowd 2018 and Norton et al.
- Conclusions. Using complete sentences, state only conclusions directly supported by the study. Avoid speculative observations but indicate the extent to which additional research may be required to address the central issues raised in the article.
After the abstract, provide five to seven keywords. The US National Library of Medicine's Medical Subject Headings (MeSH) site may be helpful.
2.4.2.3 Callout Box: What is known on this topic/What this study adds. HSR uses a callout box in each article to provide our readers with a quick summary of what is known and what your study adds to the field. Write 1 to 3 short, specific, jargon-free key points for each section, with no citations, and with a practitioner or policymaker audience in mind.
- What is known on this topic. Using no more than 3 bullet points of no more than about 30 words each, state for a broad audience what was known about the topic before you did your study and why your study needed to be done.
- What this study adds. Using no more than 3 short bullet points of no more than about 30 words each, explain what your study findings add to the field. In other words, give a simple answer to the question “What do we now know as a result of this study that we did not know before?” Include only study findings. You may also include policy or practice implications that are supported by your findings. The last bullet point should be a broadly accessible statement of the study's main take-home message.
2.4.2.4 Main text. We recommend the following standards for the main text.
- To the extent possible, we expect all Research Articles and Research Briefs to follow the appropriate EQUATOR Network guidelines. Given that the majority of manuscripts submitted to HSR apply observational designs (e.g., natural experiments) to routinely collected health data, the Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement is often applicable. We also support other consensus-based methodologic standards, including the CONSORT statement for randomized controlled trials (and extensions for cluster randomized trials), MOOSE standards for meta-analyses of observational studies, PRISMA standards for systematic reviews and other types of meta-analyses, the STARD statement on studies of diagnostic tests, and the STROBE statement on observational epidemiologic studies. Authors are encouraged to adhere to these standards whenever possible.
- Authors of qualitative papers should adhere to the Consolidated Criteria for Reporting Qualitative Research (COREQ) or the Standards for Reporting Qualitative Research (SRQR) and follow the NICE checklist, addressing each point to ensure the rigor and transparency of their methods and reporting.
- For reporting findings about or discussing race and ethnicity, we recommend that authors follow the AHA/ASA Journals Disparities Research Guidelines and JAMA guidance on reporting of race and ethnicity and CDC Preferred Terms.
We expect Research Articles and Research Briefs to use the following main section headings and content. Subsection headings may be used, especially for longer manuscripts, but are not essential. Methods articles may adapt this format as needed, for example omitting the Results section. Please do not number sections and subsections.
Introduction:
The introduction should include the following:
- Background on the importance of the issue or problem and a brief review of what is known about this issue or problem
- Rationale for the current study, including a brief description of the theory or conceptual framework that motivated the choice of data, variables, etc.
- Study objectives including one or more research questions and (if applicable) an explicit hypothesis statement. For evaluation studies, the intervention, program, or policy of interest must be clearly but succinctly described. Do not preview study results in the Introduction.
Methods:
For quantitative analyses, we recommend authors follow the general statistical guidelines provided by the Annals of Internal Medicine. The Methods section should also include the following:
- Study design (observational, randomized experiment, qualitative, etc.) and setting
- Source of participants or data and timeframe of data collection
- Variables (interventions, predictors, outcomes, confounders, effect modifiers, etc.) and their definitions and data sources. Please be clear about how all assessments and measurements were made, including specific ICD-10-CM/PCS or other codes used to identify cohorts and risk factors, using appendices as needed for details. To support data transparency and generalizability, we encourage authors to provide the logic or programming code for extracting data from its data source or creating analytic variables in a publicly accessible resource such as GitHub. If a method or tool is introduced in the study, including software, questionnaires, and scales, the author should state the license this is available under and any requirement for permission for use. If an existing method or tool is used, the authors are responsible for checking the license and obtaining permission. If permission was required, a statement confirming permission should be included in the Methods section
- How study sample size was determined, if applicable
- Data analytic approach and identification strategy or other description of statistical testing
- Statement indicating the Institutional Review Board that reviewed and approved the study or why there was an exemption of review, for example because the data were anonymized and publicly available or because the study was for quality improvement purposes and not intended as generalizable research
- For studies based on surveys, a report of response rate and a citation or description of analysis of nonresponse bias in Methods, Results, or an Appendix, as appropriate
- For qualitative studies, a statement of the study's analytic framework, protocol, or guidelines
- For studies that do not disclose data sources, a statement that anonymous data sources were used and documentation was confirmed by HSR editors. (Authors will receive an email requesting documentation, for editors' review only, such as a data use agreement or letter from the data source acknowledging use for research publication.)
- Description for calculations of average marginal effects and incremental effects (preferred over odds ratios except for case-control studies (see Section 2.4.2.2).
- Author statements about their background and lived experiences that may influence interpretation of their data (optional)
Mathematical equations or expressions are discouraged in the text unless they are crucial to understanding the modeling approach that was employed. They may alternatively be provided in an appendix, as part of the supplementary online materials.
Results:
The results section should include:
- Descriptive data on sample and/or participants
- Main results, highlighting key findings and referring to tables and figures as appropriate (with full model results in an appendix for reviewers, as part of the supplementary online materials, if not part of the manuscript’s tables)
- Other analyses, including robustness or falsification tests as appropriate
- Tables displaying covariate balance before or after matching or weighting should include standardized differences as a metric of balance
Discussion:
The discussion section should include:
- Brief summary of key results, preceded by as much context and design of the study so that a reader of only the Discussion can understand the what the results mean
- Compare and contrast of key results with comparable results from prior studies, including studies with both convergent and divergent findings
- Limitations of the study and efforts to address or mitigate those limitations, when appropriate, though we recommend not ending the article with limitations
- Implications of results for practice, policy, research, or other anticipated end users (with comments on generalizability to other settings, when appropriate). Please avoid generic statements about the need for additional research; specific evidence-based recommendations are preferred.
2.4.2.5 References. All references listed must be cited in the text, and all text citations must have a reference listed. HSR uses the AMA citation style. The AMA style uses numerical citations within the text and a list of these citations in numerical order, by order of appearance, rather than alphabetical order. Please do not use footnotes, but instead provide a comprehensive list of references at the end of the manuscript. Multiple references should not be combined under a single number. The list of references at the end of the document should be formatted as follows:
Journal article:
1. Chen LM, Ryan AM, Shih T, Thumma JR, Dimick JB. Medicare's acute care episode demonstration: effects of bundled payments on costs and quality of surgical care. Health Serv Res. 2018;53(2):632-648.
2. Mukamel DB, Amin A, Weimer DL, et al. Personalizing nursing home compare and the discharge from hospitals to nursing homes. Health Serv Res. 2016;51(6):2076-2094.Book:
1. Dusetzina, SB, Tyree S, Meyer AM, Meyer A, Green L, Carpenter WR. Linking Data for Health Services Research: A Framework and Instructional Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014.
Website and software references:
1. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. Supplement 1. Study protocol and changes to analysis plan. JAMA. 2016;315(6):562-570. Accessed June 18, 2019. http://www.jamanetwork.com/journals/jama/fullarticle/2488307
Internet document:
1. Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE tables. Published December 3, 2015. Accessed June 14, 2016. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html
2.4.2.6 Tables (each with title and footnotes). Each table should be on a separate page in the file and must be specifically cited within the text using Arabic numerals. Number tables in the order they appear in the text. Keep titles brief but clear and descriptive of the contents. Avoid titles such as “Regression Results,” as they convey nothing about the table content. Tables should be self-contained and complement, not duplicate, information in the text. They should be supplied as editable pages or files, not pasted as images. Use a separate cell for each datapoint (with related information such as percent of total or confidence intervals, as appropriate). Footnotes should be concise but comprehensive.
The table and footnotes must be understandable without reference to the text. All abbreviations must be defined in footnotes, even if they are defined elsewhere in the paper. Use superscript letters for linked table footnotes (a, b, c, …), with asterisks reserved for statistical significance, for example: ***, **, and * denote statistical significance at the 1%, 5%, and 10% levels. Column or row headings should specify when statistical measures (such as percent, CI, SD, SEM, or P values) are used.
While tables may be of any length, include only tables and content therein necessary for the reader to understand and evaluate the main findings. Additional information should be in an Appendix. Authors of manuscripts with unreasonably large tables may be asked by editors to move some of the content to an appendix.
2.4.2.7 Figures and figure legends. Figures can be in the main document or one or more separate files. Please review the figure requirements for peer review and post-acceptance manuscripts.
Although authors are encouraged to send the highest-resolution figures possible, for peer review, a wide variety of formats, sizes, and resolutions are accepted so long as they are readable by all. Text in figures must be legible when the page is viewed at 100%. All illustrative artwork (figures) must be original.
While figures may be divided into panels, the spirit of the limits indicated in Section 2.3 should be observed. We recommend that all panels of a single figure fit on one page, with sizes that allow readability by all. Authors of manuscripts with unreasonably large numbers of panels per figure may be asked by editors to move some of the content to an appendix.
Figure legends must be in text, not as footnotes or embedded in figures as part of the image. Figures and legends must be understandable without reference to the text. Labels on the axes of graphs must be meaningful to readers. Legends should be concise but comprehensive. Include definitions of any symbols used and define/explain all abbreviations and units of measurement, even if they are explained in the main text.
2.4.2.8 Supplementary files
Supplementary files for publication should be uploaded as separate documents from the main manuscript text. Any information for editors or reviewers that is not for publication can be stated in the cover letter and the editorial staff will contact you if we need documentation (such as a paper under review that describes a study sample).
Examples of supplementary files include appendices with details on the questions used in a survey or a database, inclusion and exclusion criteria, or alternative modeling results. Upon publication, this information is hosted online and appears without editing or typesetting. It may include text, tables, figures, audio files, video files, datasets, etc. Wiley has FAQs on supporting information, including acceptable file formats. There is no limit on the number of supplementary files that can be made available to readers, but please restrict individual file sizes to 10Mb or fewer (zipped or unzipped) as larger files can lead to download issues for users. Label all files clearly as "Supporting Information" (e.g., use SuppInfo, Supp, or Supplemental in the filename). Include a text legend, preferably close to the figure or other supporting material, explaining what it is. Supporting or supplemental material should be cited in the main text of the manuscript
If data, scripts, or other materials used to generate the analyses presented in the paper are on a publicly available data repository, authors should include a reference to the location of the material within their paper and do not need to separately upload it as a supplemental file. If an article contains copyrighted material reproduced from other sources, written permission from the author and publisher to use such material must be received prior to our sending the manuscript for review.
-
HSR uses the AMA Manual of Style. (Subscription is required for access. Your institution's library may have access. Authors who lack access can submit style questions to hsr@aha.org.) The following points provide general advice on formatting and style.
- Abbreviations: In general, terms should not be abbreviated unless they are used repeatedly and the abbreviation is helpful to the reader. Initially, use the word in full, followed by the abbreviation in parentheses. Thereafter use the abbreviation only. We discourage the use of abbreviations that are not already in common use, or that may have more than one meaning. Abbreviations used in the abstract, callout box, main text, tables, and figures should be separately defined in each.
- Spelling and grammar: Please use spell-checking and grammar-checking features in your word processing software to minimize errors before submission. HSR uses US spelling; however, authors may submit using either British or US spelling, as spelling of accepted papers is converted during the production process.
- Numbers: Numbers under 10 are spelled out, except for measurements with a unit (8 mg/day); numbers in statistical or mathematical functions; numbers that represent scores, points on a scale or series, or time including age (6 weeks old); or lists with other numbers (11 hours, 9 years).
- Trade names: Chemical substances should be referred to by the generic name only. Trade names should not be used. Drugs should be referred to by their generic names. If proprietary drugs have been used in the study, refer to these by their generic name, mentioning the proprietary name and the name and location of the manufacturer in parentheses.
- Proper names of languages, peoples, races and ethnicities (e.g., Black, White, Latina): Capitalize according to AMA style.
- Race and ethnicity terms: Use terms preferred by study participants. Include country or region of origin if possible. If asking participants is not possible, for example when using electronic health records, Medicare or Medicaid datasets, or surveys, use the dataset's prespecified terms. For example, ask participants if they prefer Hispanic, Latino and Latina, Latina/o, or another term such as Latinx. However, if using data collected using a specific term such as Hispanic or Latino, then use that term in your manuscript.
- Updates: As preferred usage changes, we will update these guidelines. For reporting findings about or discussing race and ethnicity, we recommend that authors follow the AHA/ASA Journals Disparities Research Guidelines and JAMA guidance on inclusive language and CDC Preferred Terms.
-
At submission, the corresponding author, on behalf of all authors, will attest to authorship as defined by the International Committee of Medical Journal Editors (ICMJE). All qualified authors should have the opportunity to participate in drafting, review, and final approval of the manuscript.
At submission, the corresponding author will also disclose potential conflicts of interest (defined in the ICMJE Disclosure Form) of any manuscript author and prior dissemination. As long as it is disclosed, the existence of a possible conflict rarely precludes publication. Financial and material support for the content of the manuscript must be disclosed in the Acknowledgments section (2.4.2.1) and prior dissemination described in the cover letter.
For accepted manuscripts, we will publish a statement recognizing the forms of support that made the project possible, based on information provided at submission.
